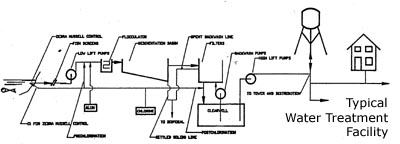
Water in the natural environment contains many contaminants which make it unsuitable for human consumption. These contaminants include pathogenic microorganisms, colour, odour, suspended matter and chemicals which either endanger public health, are unappealing to the senses, or casue engineering problems. Treatment processes remove or decrease the contaminant levels so as to make the water fit for human consumption. The process described below is a typical process used in Ontario when water is drawn from a relatively clean body of surface water. The figure below illustrates the flow of water through a treatment facility. Groundwater supplied systems may have simpler treatment facilities due to the quality of the source water.
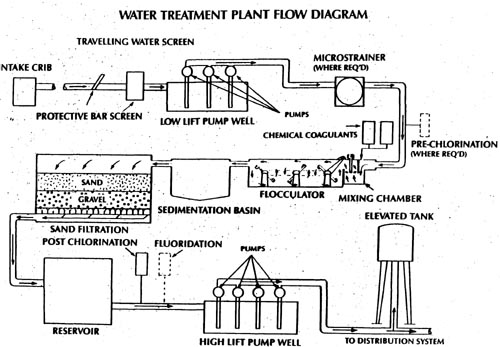
Raw water will contain small particles, known as colloids. These colloids produce a cloudy appearance known as turbidity. Turbidty can shield microorganisms from disinfection. Colloidal particles either do not settle or are very slow to settle. For this reason chemcials are added which will react with the colloids and the water to form relatively large, easily settleable particles. The mixing of these chemicals (coagulants) with the water is known as coagulation.The reaction between the coagulant and the particles is very fast and thus the chemicals must be mixed quickly and thoroughly in the water to assure that it has achieved maximum effect. For this reason the process is called rapid or flash mixing.The most commonly added chemical for this purpose is aluminum sulphate (Alum). Ferrous sulphate, ferric sulphate and various ploymers are also commonly used coagulants. The water then passes into a flocculation tank. In the flocculation process the water is mixed slowly and the particles (floc) build in size and density. The purpose of flocculation is to achieve optimum size particles which will readily settle out of the water.
The addition of too much or too little coagulant decreases the efficiency of the treatment process. For this reason it is very important to determine the correct dosage. The optimal chemical dose is determined in the laboratory using a "jar test" which simulates the conditions in the plant on a small scale.
From this point the water normally flows into sedimantation tanks (or clarifiers) wherein the heavier matter settles due to gravity. The time the water remains in these tanks is known as detention time. The settled material is usually disposed of in the sanitary sewers. Surface waters may also require a pre-sedimentation period, before any other treatment process in order to remove very large material, such as sand and organic debris.
The next step in the process is filtration. Removing as much suspended particles as possible is very important for the operation of a treatment facility. Not only will the water appear clean to the consumer, but removing suspended particles eliminates the possibility of dangerous bacteria being protected from the disinfection process. The most common type of filtration process is known as the rapid sand filtration. Although there are various types of various sand filters, most operate using the same general principles. These filters normally contain layers of anthracite, sand, and/or gravel. The tiny pore spaces in the filter "media" trap the suspended particles. Most matter which has not settled in the flocculation tank is entrapped by the filter media. Activated carbon may also be used to remove taste and odour problems. The quality of the water, as it leaves the filtration process can be determined by its turbidity. High tubidity may indicate that the filters require cleaning.
Filters are cleaned on a regular basis (usually 1-4 days depending on the facility) by a process known as backwashing. During this process the normal water flow is shut off and fully treated water is pumped up, backwards, through the filter media to remove the entrapped matter. The backwash water will be further treated to remove the solids or routed to the sanitary sewer.
Before the water is distributed to the community one or more chemicals may be added to help disinfect, adjust pH, or remove other impurities. The most common addition to chlorine, used, primarily, to kill harmful microorganisms in the water. Chlorine, when used as a disinfectant, is added after solids removal but at a point which will allow complete mixing. It is necessary to ensure that the chlorine has sufficient time to react with the water and the microorganisms (this period of time is known as the chlorine contact time). For this reason, the water and chlorine willbe allowed to react in a chlorine contact chamber or clearwell. In this chamber the chlorine residuals are raised to the required standards. Other disinfection methods such as ozonation and ultraviolet radiation (UV), are also used.
Taste and odour in drinking water is a common consumer complaint. By themselves taste and odour do not cause any health problems. To encourage consumer usage it is important for treatment facilities to minimize their impact. Several methods are in common use to combat taste and odour including aeration (i.e. mixing air in water), addition of chemicals such as chlorine or potassium permanganate, and the use of activated carbon. Generally, water of high oxygen content and low temperature produce a superior tasting water.
Other compounds such as iron and manganese may also be treated if their concentration is too high. The most common methods of treatment are aeration, chlorination, addition of chemicals or ion exchange. Many treatment systems will also add fluorine to the water to prevent tooth decay. Chemicals may also be added to adjust the pH of the water. pH adjustment is often necessary to optimize the coagulation process. Waters which are corrosive in nature may also be treated chemically before distribution.
After the water is treated it flows to a large tank, normally called a clearwell or chlorine contact chamber, where it is pumped to the distribution.